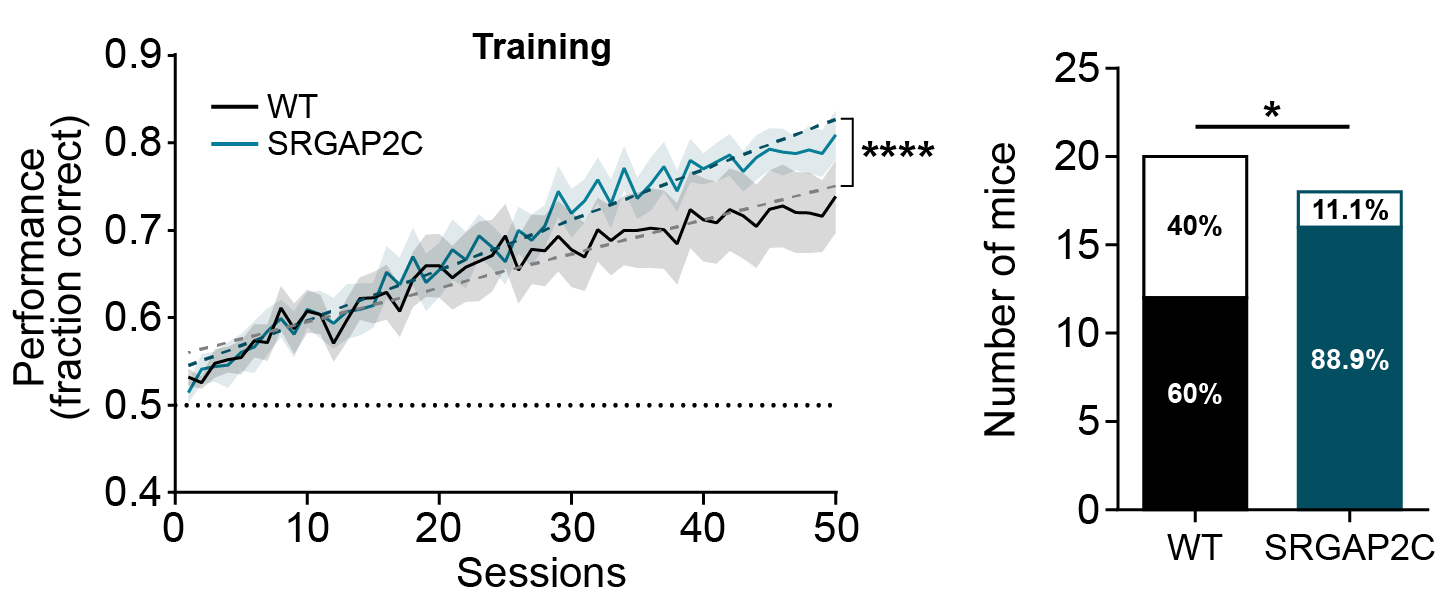The structural organization of neuronal circuits is a critical determinant of brain function. It is this wiring diagram that determines how information flows through the brain. Indeed, alterations in circuit connectivity can profoundly modify how the brain functions.
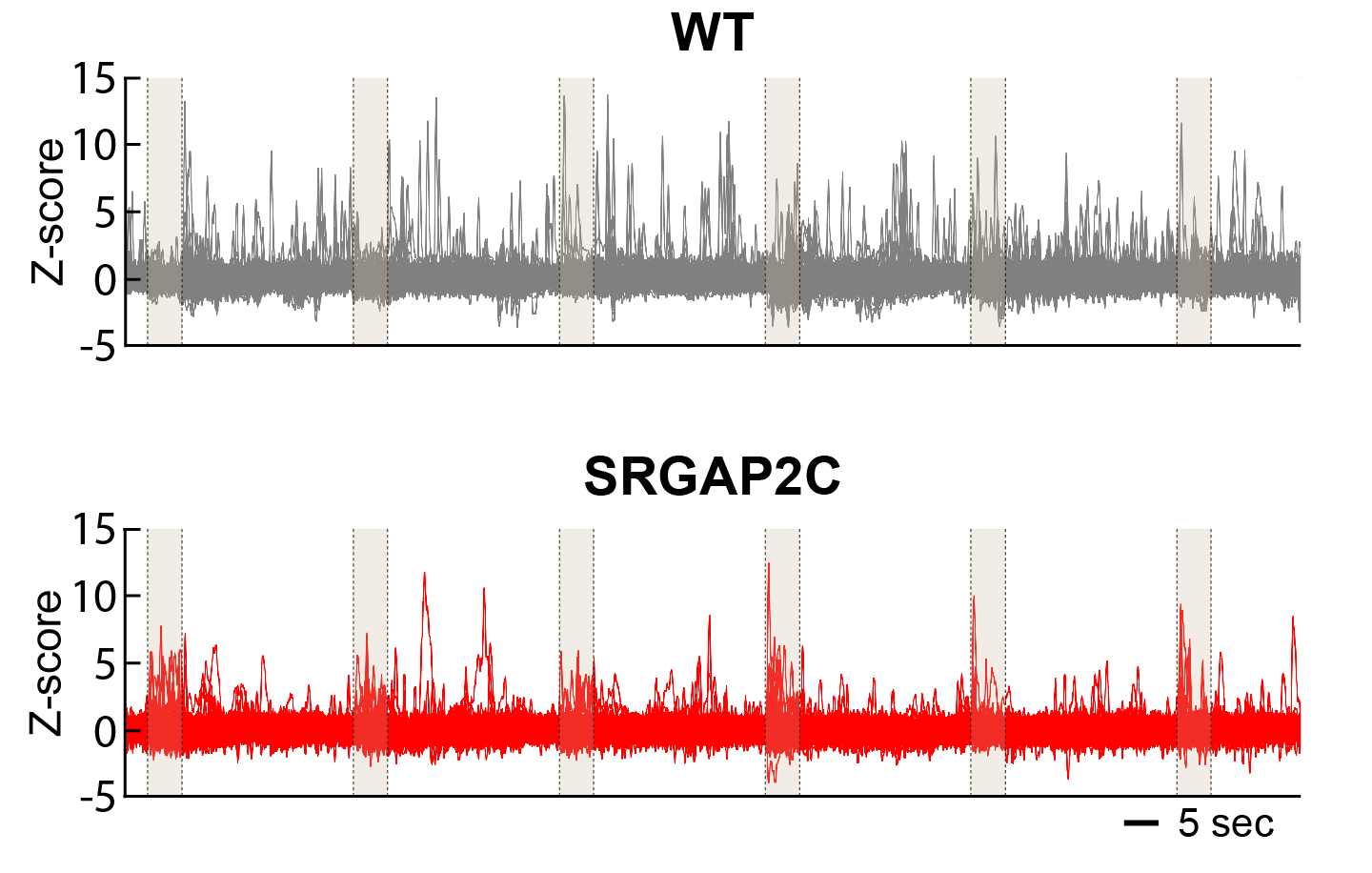
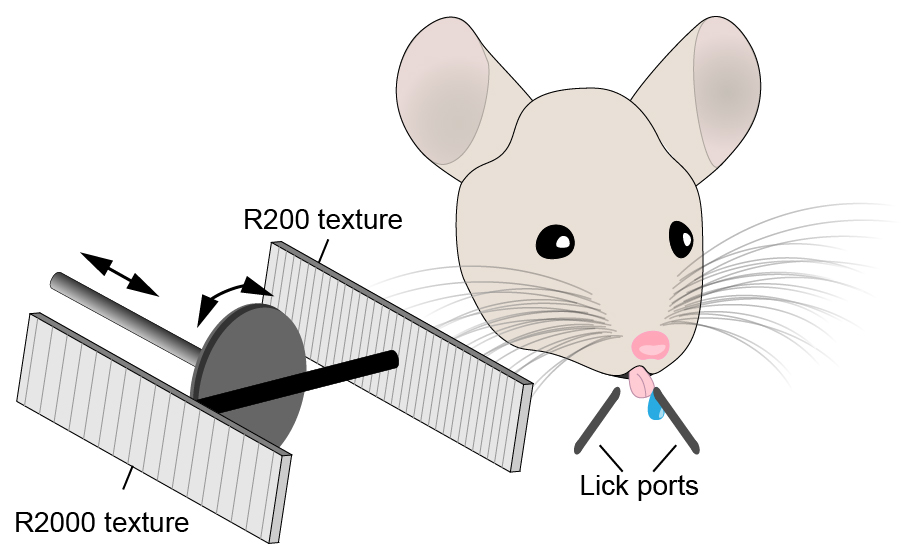
Synapses are the point of contact where inputs from other neurons (presynaptic) connect with the target neuron (postsynaptic). Changing how synapses develop can therefore have significant impact on how neurons connect with each other. Our previous work has shown that the human-specific gene (HSG) SRGAP2C uses this mechanism to modify cortical connectivity. By modifying synaptic development, SRGAP2C changes cortical circuits by selectively increasing feedforward and feedback cortical connectivity – projections that are considered critical for human cognition.
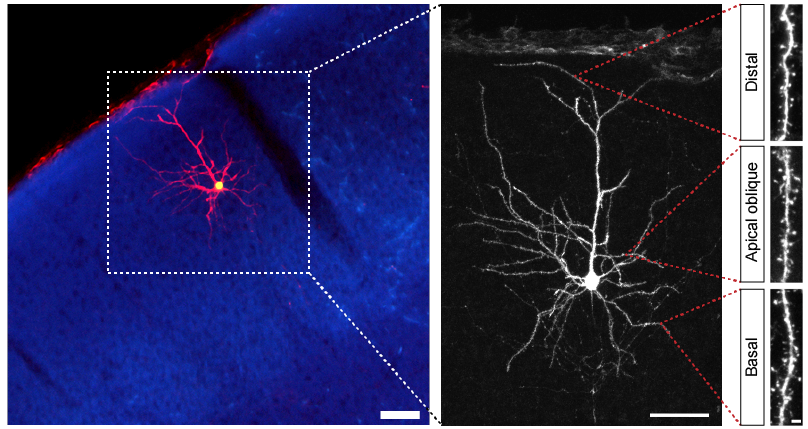
Using sparse in utero electroporation techniques we target individual neurons and visualize their morphology in high detail, up to individual synapses (e.g. dendritic spines as shown on the right). This enables us to determine whether the number and organization of inputs onto these neurons has changed, for example in response to impaired synaptic development or the expression of HSGs.
We made these discoveries by using a combination of in utero electroporation and viral transduction techniques that enables us to visualize individual neurons and subcellular features, such as dendritic morphology and synapses. This allows us to study if and how the organization of neuronal inputs has changed.
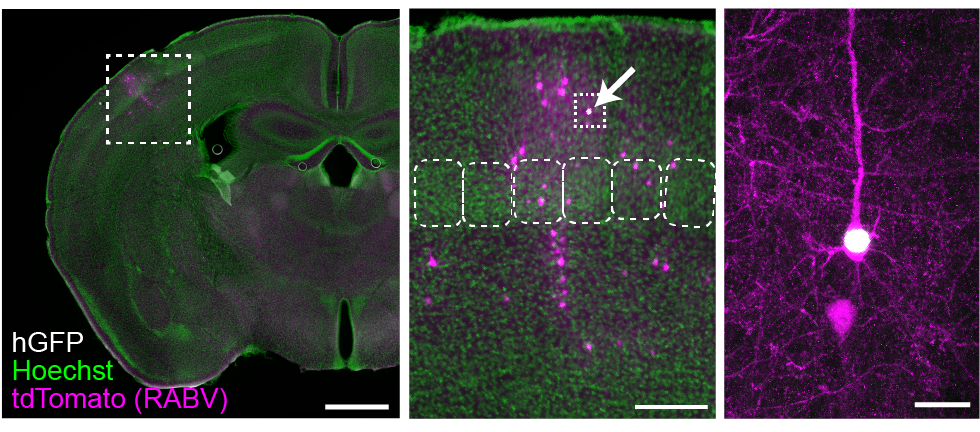
Sparse in utero electroporation and viral tracing techniques, such as monosynaptic rabies tracing, enables us to map the connectivity profile of individual neurons. Visualized here is the presynaptic connectivity (neurons in magenta) for a specific cortical neuron (expressing hGFP) in the mouse primary somatosensory cortex.
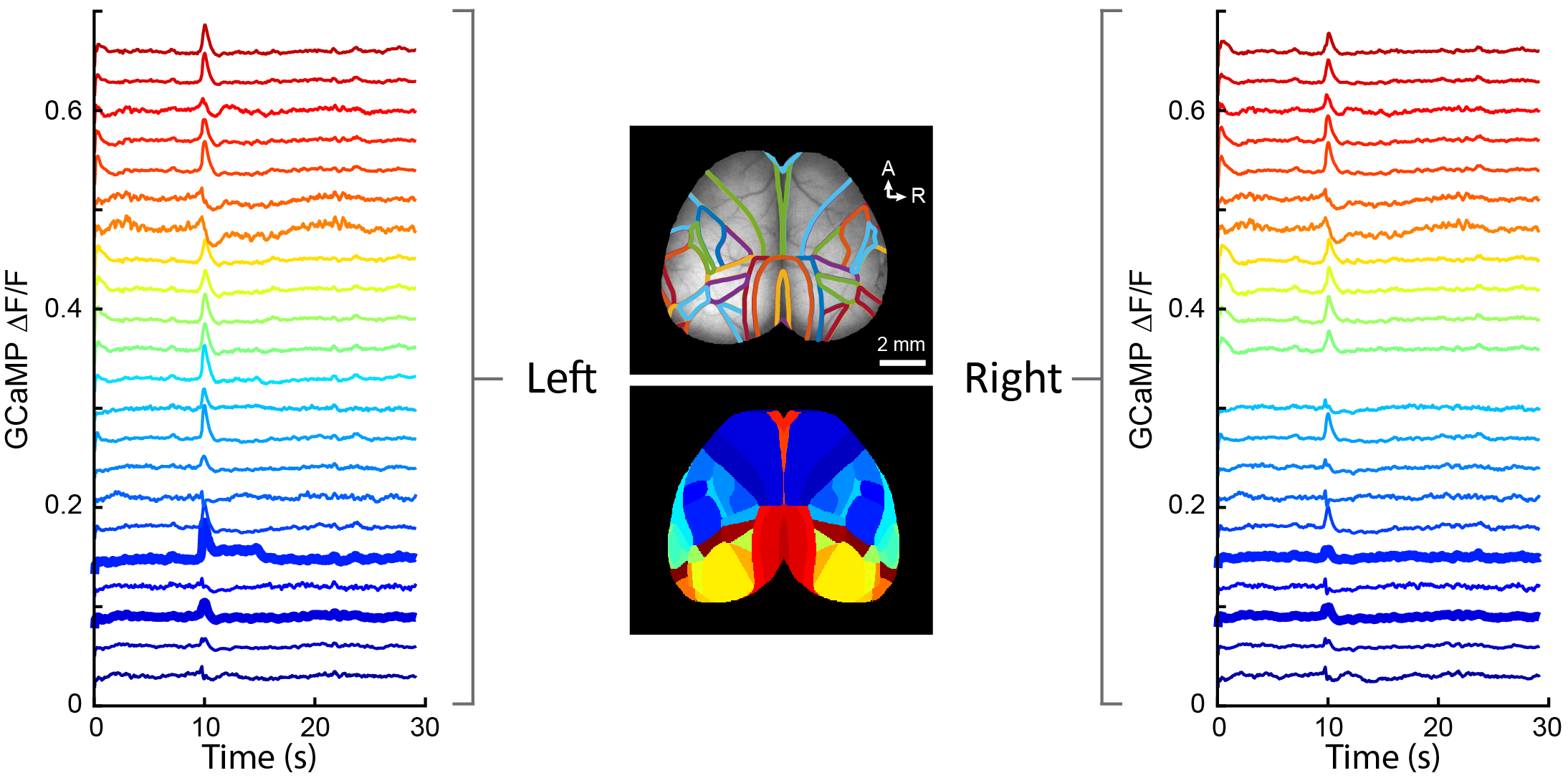
In addition, using a variety of tracing techniques, including whole-brain monosynaptic rabies tracing, we map connectivity across the entire brain and assess how HSGs alter the structural organization of neuronal circuits. These approaches also enable us to determine how impaired synaptic development, as observed in neurodevelopmental disease such as Autism Spectrum Disorder, alters the structural organization of neuronal circuits throughout the brain.
Using a method for whole-brain reconstruction and registration of every traced neuron onto the Allen Brain Atlas, we identify the anatomical location of each traced presynaptic neuron that connects with a given cortical neuron (starter neuron). This enables us to quantify how impaired synaptic development or the expression of HSGs modifies the organization of neuronal circuits across the brain.